Summary:
- Cells use a variety of mechanisms to regulate proteins.
- Amgen’s Ray Deshaies and Yale’s Craig Crews teamed up to harness one of these systems to degrade disease-causing proteins with the development of small molecules called PROTACs.
- Amgen’s Induced Proximity Platform (IPP) capitalizes on this idea to create molecular “matchmakers” that link target proteins with natural regulatory systems in the body that can potentially inhibit or eliminate those targets.
When Ray Deshaies met Craig Crews, he had no idea they would go on to develop a potential new way to target disease. At the time, Deshaies, now senior vice president of Research at Amgen, was studying a family of enzymes called ubiquitin ligases. These enzymes act as a cell’s protein quality control system, tagging unwanted proteins for destruction in the cell’s protein degradation machine, the proteasome. Deshaies’ lab at the California Institute of Technology had recently discovered a new member of this family. Crews, the John C. Malone Professor of Molecular, Cellular, and Developmental Biology at Yale University and his lab were working on a way to connect proteins together with small molecules so those proteins could carry out novel functions.
The two met for the first time in 1998 at a retreat for recipients of the Burroughs-Wellcome new investigator award in the basic pharmacological sciences. Deshaies noticed Crews’ poster at the retreat, and they started talking. Crews was intrigued.
”The conversation started at Craig’s poster and there was a little bar nearby. So we ended up continuing to talk over beers,” said Deshaies. They landed on an intriguing idea: What if they could use Crews’ protein-protein bridge work to guide Deshaies’ enzyme that marks proteins for degradation to target disease-causing proteins?

Yale University’s Craig Crews (left) and Amgen’s Ray Deshaies at the 2023 Gabbay award ceremony, where they were award co-recipients for their work on PROTACs, or proteolysis-targeting chimeric molecules.
A system used by viruses, plants and even a blockbuster drug
Viruses, like Human Papilloma Virus (HPV), Human Immunodeficiency Virus (HIV) and others have been gaming the cellular ubiquitin-proteasome system to outsmart their hosts for millions of years. They hijack this system to eliminate the host proteins that are trying to block them.
Deshaies reasoned that what he and Crews were attempting must be a powerful approach if viruses evolved to exploit the same idea. Years later, this hunch was confirmed by work in plants, which showed that they use a hormone called auxin that links the ubiquitin–proteasome system to growth-regulating targets. “That’s when I realized this idea is bigger than we originally thought,” said Deshaies.
But it didn’t end there – several years later, other researchers discovered that lenalidomide, a chemotherapy for the blood cancer multiple myeloma, acts through the same mechanism that viruses and plants use. A version of the drug, known as thalidomide, had been around since the 1950s, when it was prescribed to treat nausea in pregnancy. After it was discovered to cause birth defects, its derivative, lenalidomide, was found to work in multiple myeloma and became a blockbuster medicine.
Researchers later determined that lenalidomide targets a transcription factor, a protein that regulates the transcription of genes from DNA to RNA, that is then translated into a protein. Transcription factors have been notoriously difficult to target. Knowing that lenalidomide was able to target transcription factors for degradation generated a lot of interest among drug hunters.
Introducing: The PROTAC
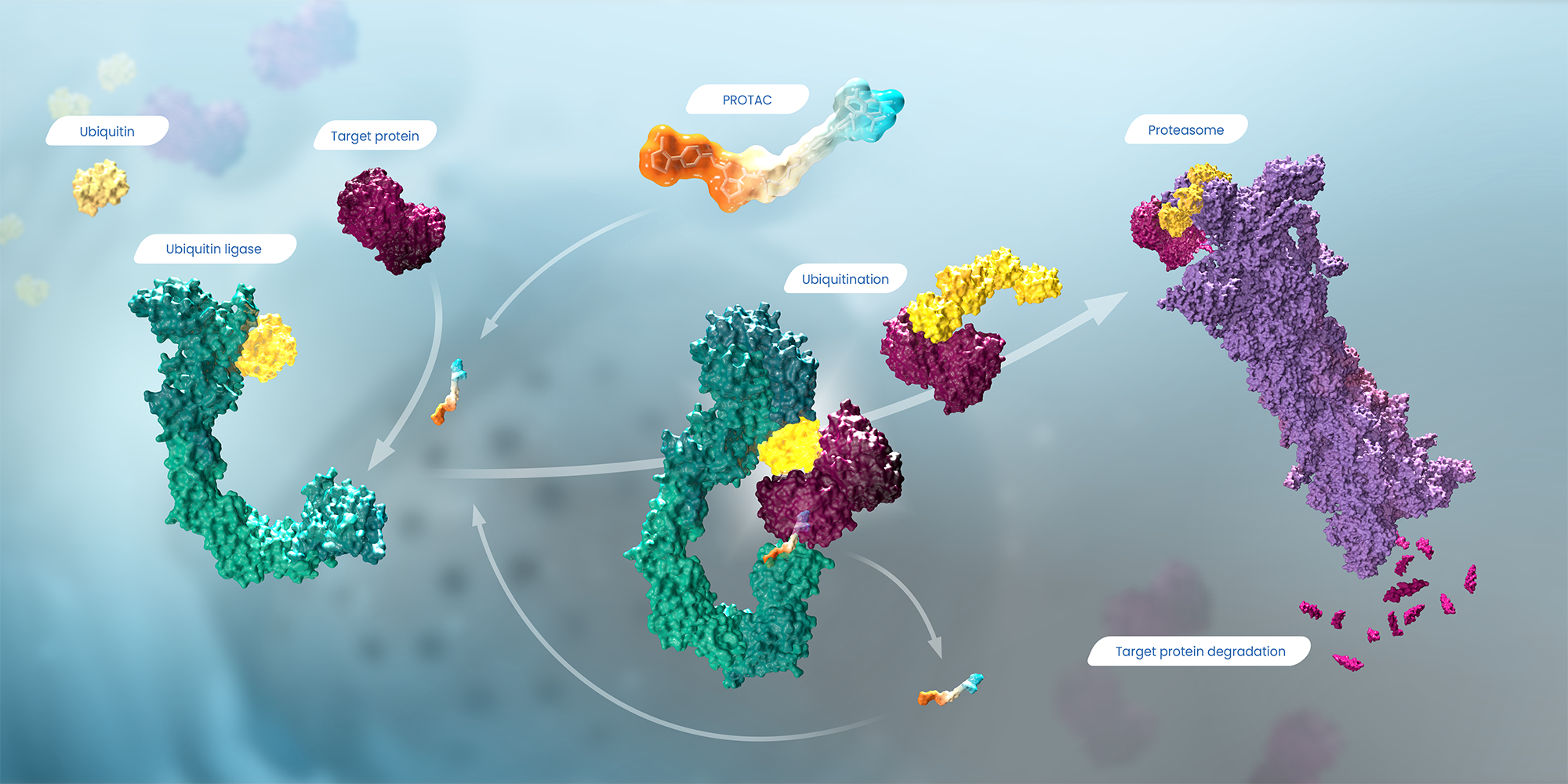
Spurred by the recent discoveries on the ubiquitin-proteasome system, Deshaies and Crews came up with their first proof-of-concept molecule that they called a PROTAC, or proteolysis-targeting chimeric molecule. Acting like a molecular “matchmaker,” a PROTAC is a dual binding, or bispecific, small molecule that has one “hand” that grabs a protein target of interest. Its other hand latches on to a ubiquitin ligase, the enzyme Deshaies’ lab was studying. Once matched to the target, the ubiquitin ligase tags it with ubiquitin molecules so it is recognized and degraded by the cell’s proteasome.
Unlike a conventional small molecule drug that must stay bound to its target, the Crews lab has shown that a PROTAC can act on a target and then move on to the next one to act again. Also, rather than simply altering the target, a PROTAC gets rid of it entirely, potentially minimizing the possibility of drug resistance.
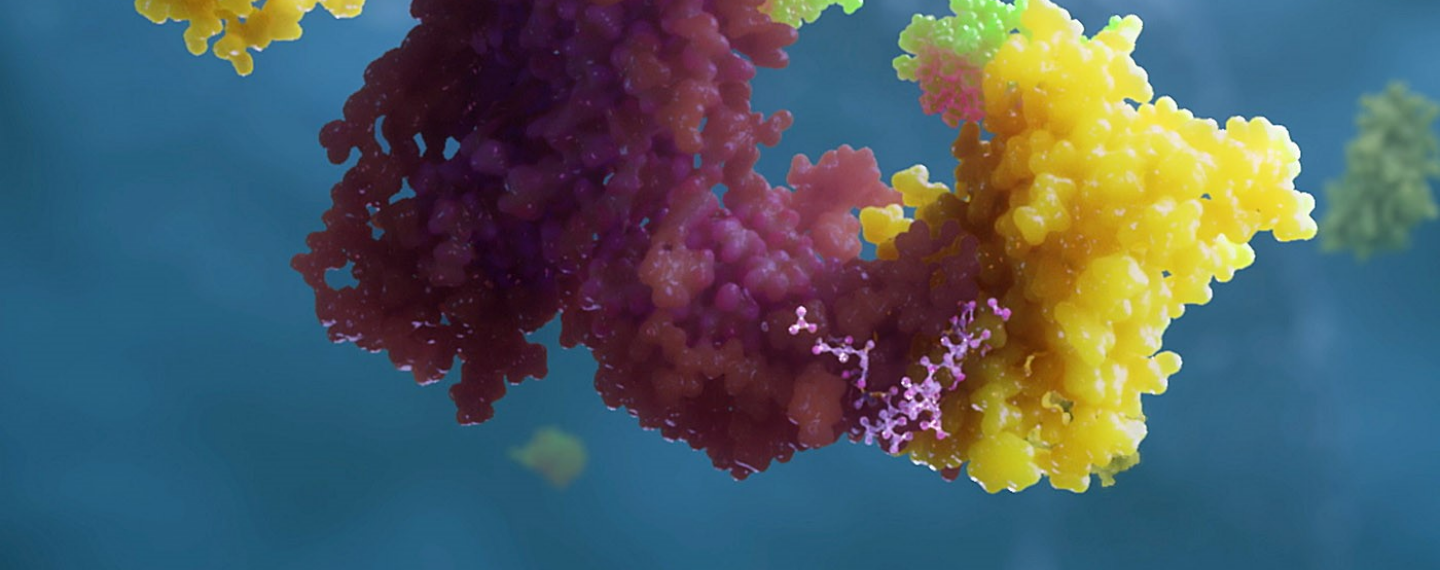
A PROTAC molecule (at top, middle) is like a piece of double-sided tape that sticks to both a ubiquitin ligase (at left, in green) and a target protein (in dark purple), joining them together. Ubiquitin (in yellow) adds a chain of ubiquitin molecules to tag the target so it is recognized by the proteasome (at right, in light purple), which then degrades the target protein into small peptides. Click image to enlarge.
After developing their first PROTAC, Deshaies and Crews set their sights on engineering two new PROTACs that successfully targeted and led to the degradation of both the estrogen receptor, associated with progression of breast cancer, and the androgen receptor, associated with prostate cancer progression.
But getting their PROTACs inside of cells, where many disease targets are found, was still a challenge. During a Howard Hughes Medical Institute (HHMI) meeting, Deshaies met fellow HHMI researcher, William Kaelin, who was also exploring the ubiquitin-proteasome system. Kaelin, who is a professor at Harvard University and later won a Nobel Prize for Medicine for his work on how cells sense oxygen, described a molecule he was studying that bound a particular ubiquitin ligase and could enter cells. Deshaies and Crews built on this insight to develop a new suite of PROTAC molecules that could enter into cells to trigger degradation of target proteins.
Deshaies and Crews’ foundational work on PROTACs captured the attention of the scientific community, including being named co-recipients of Brandeis University’s 25th Jacob and Louise Gabbay Award in Biotechnology and Medicine.
Targeting the undruggable by inducing proximity
At Amgen, Deshaies has helped set up a formal research program to study molecules like PROTACs called the Induced Proximity Platform (IPP). Led by Ryan Potts, vice president of Research, the goal of the IPP is to come up with new therapeutic approaches to target the 85% of proteins and other molecules that are considered undruggable. Instead of having to bind the target and act on it directly, an IPP molecule can bring the target in close proximity to systems in the cell like the ubiquitin- proteasome system for degradation.
The IPP is focused in part on degrading proteins through PROTACs, which have the potential to work with hundreds of different types of ubiquitin ligases, as well as smaller versions of PROTACs known as molecular glues. Both PROTACs and glues work to match protein targets with ubiquitin ligases for destruction. Although they elicit the same end result, PROTACs are more modular and easier to discover, but are bulkier than drug-like molecular glue degraders.
One current IPP project is looking to target a protein called SMARCA2 that is found in certain forms of lung cancer. Lung cancer cells that have a mutant version of SMARCA4 that no longer functions properly depend on its near-identical twin SMARCA2 for survival. The hope is that developing a PROTAC that selectively targets SMARCA2 for degradation could block lung tumor cell growth while sparing normal cells.
The ability to harness the power of the ubiquitin-proteasome system is just the tip of the iceberg. Researchers in the IPP group are also looking to target molecules such as RNA through development of RNATACs, or RNA-targeting chimeric molecules. These work similarly to PROTACs but bind to RNA molecules instead of proteins and bring them to RNA degrading enzymes for destruction. Targeting RNAs that go on to make proteins eliminates the need to bind to a potentially undruggable protein.
Another area of induced proximity research includes LYTACs, which bring targets to the lysosome, an additional way cells degrade molecules. But unlike the proteasome that degrades proteins primarily located inside the cell, lysosomes can degrade proteins that are located both inside as well as outside of cells. This includes important signaling molecules secreted from cells or on the surface of cells.
As researchers continue to study the biology of these types of systems, it’s clear there is huge potential to match targets with the many approaches cells use to control their proteins. “This idea of inducing proximity is not just about degraders, since you can potentially induce any sort of proximity to change the function of a target protein,” said Deshaies. ”It's possible this ends up being the most important way of developing small molecule drugs in the future.”