Show Transcript
If scientists can deliver vaccines for COVID-19 in under a year, why does it normally take a decade or more to develop other types of medicines? There's a long way and a short way to answer that question.
The long way is to describe the myriad steps in the R&D process and the years of testing needed to show a drug's benefits outweigh its risks. The short answer is drug development takes too long and needs to move faster. Much faster.
Even before the pandemic showed what science could do when spurred by a global crisis, speed had emerged as a top strategic priority for Amgen's R&D group. "Historically, it takes 12 to 14 years on average for a drug to go from an idea in the laboratory to an approved medicine that's available to patients," said David Reese, executive vice president, R&D. "Amgen has already reduced its drug development timelines by about three years, and there is still ample room for improvement."
"We're focused on diseases where patients' lives are literally at stake," said Rob Lenz, senior vice president, Global Development. "When you're facing an illness like cancer or heart disease, you don't want therapies 20 years from now—you want them now."

David Reese, executive vice president, Research and Development
That urgency felt by patients can prompt drug development teams and regulators to work together to expedite breakthrough therapies. A case in point is a recently launched Amgen medicine for lung cancer that received FDA approval just 33 months after its entry into the clinic. While examples like this show it's possible to dramatically accelerate promising programs, Amgen's broader goal is to make speed the norm and not the exception across its clinical pipeline.
"We are applying speed levers in multiple areas, from digital technology and innovative study designs to regulatory partnerships," said Kimberly Clemenson, vice president, R&D Transformation. "Our goal is to help each drug development team integrate as many of these levers as possible to accelerate progress."
Balancing priorities
Speed in R&D is intertwined with other priorities that place limits on how fast a drug can advance. There are strict limits that pertain to patient safety and regulatory compliance. No potential therapy can move forward without meeting these bedrock commitments.
Speed is also constrained by the need to manage the high cost of drug development and improve success rates. The estimated cost to deliver a new medicine has risen to $2.6 billion, a sum that reflects the expense of the roughly 90% of clinical programs that fail to yield an approved therapy.
"To increase success rates, teams can accrue more data at each stage of development, but that makes the program go slower," said Lenz. "Optimizing for cost by staging your investments also slows you down, while optimizing for speed tends to drive up the cost."
One way to manage these tradeoffs is to prioritize speed over cost by investing aggressively. This approach makes sense if there's strong evidence that a test drug is likely to succeed in the clinic and help patients with serious illness who have few if any good treatment options.
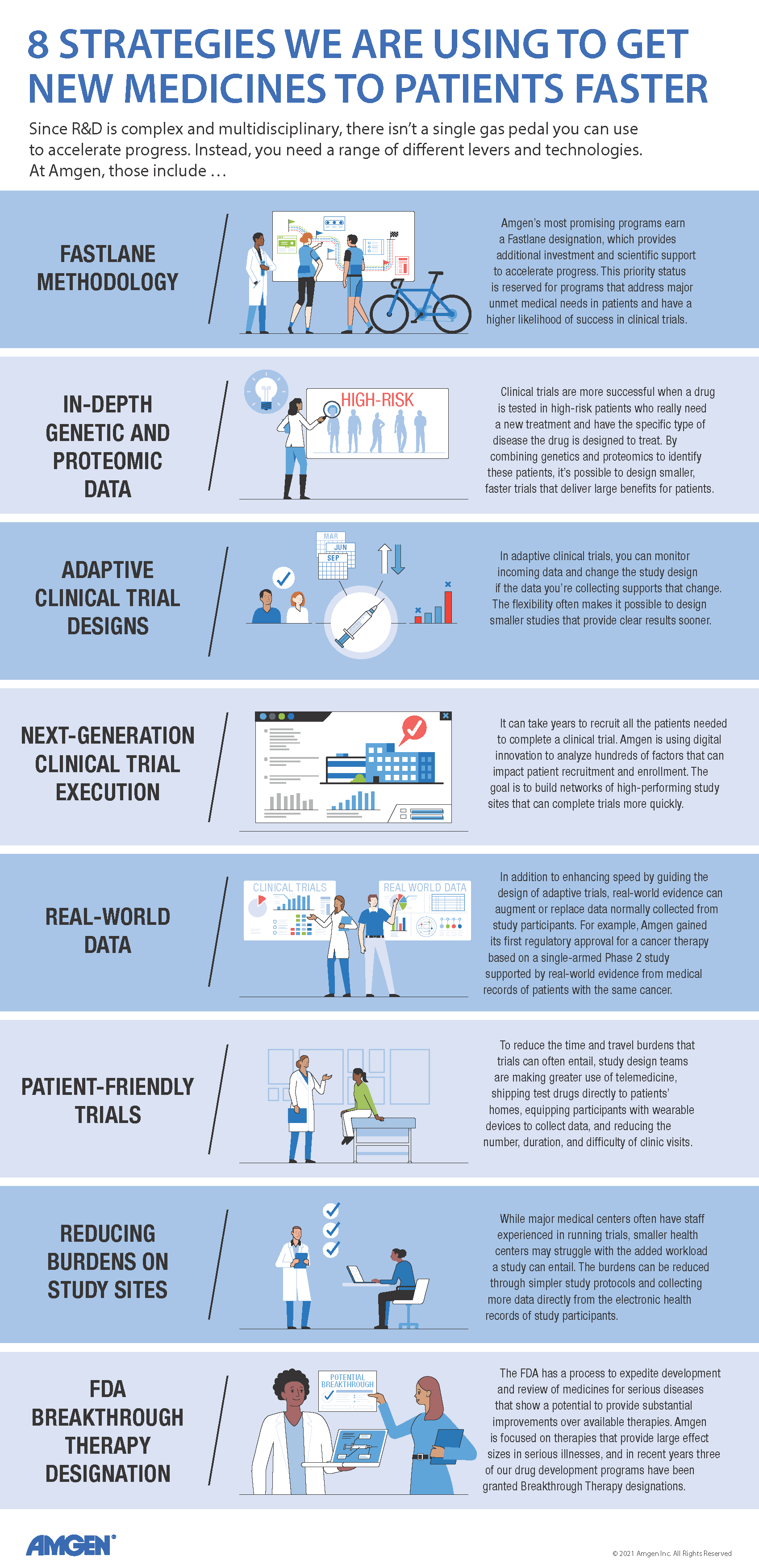
At Amgen, programs with exceptional promise can earn a "Fastlane" designation. "With the Fastlane approach, you identify the theoretical minimum time needed to advance a drug, and you do everything you can to achieve that level of speed," said Tara Arvedson, executive director, Research.
For example, in a typical drug development program, you delay high-cost activities—like creating large batches of test drug for clinical trials—until you're sure the drug will actually make it into the clinic. In a Fastlane program, you plan for success and do more work in parallel versus sequentially. More staff are assigned to Fastlane programs, and they get first dibs on support from scientists who assess a molecule's safety and other key attributes. Preparation for clinical trials also starts earlier to eliminate "white space," or the planning time that is usually sandwiched between one stage of development and the next. Together, these approaches can trim up to five years or more off the development time for a successful medicine.
But not every program can get top-priority treatment. "The Fastlane approach is reserved for programs where we have the greatest conviction or see the greatest need," said Clemenson. "That's why we're exploring other speed levers that could be applied more broadly across our entire portfolio of products."
Innovation in the clinic
Since most of the time required to develop a drug is consumed by clinical trials, many speed levers are aimed at getting answers more quickly in the clinic. They include adaptive clinical trials and other novel study designs, applying genomics and proteomics to identify patients who are more likely to need a test drug and show clear benefits, and greater use of real-world evidence and data to replace some or all of the data that is normally collected through a clinical trial.
"With adaptive designs, you can monitor the incoming data and modify the protocol based on what you're learning as the study unfolds," Lenz noted. If pre-established criteria are met, a range of adaptations can be made, including dropping or adding doses, increasing the size or duration of the trial, and observing which types of patients the drug really helps and enrolling more of them.
In general, this flexibility makes it possible to design smaller studies that deliver clear results in less time. "The sooner we can be confident that a drug or a dose of a drug either works or doesn't work, the sooner we can either advance that drug or stop evaluating it in patients," said Lenz.
Genetic and proteomic data can help solve a problem that inflates the size and duration of many studies. Just as an aspirin can't relieve a headache in someone who doesn't have a headache, a test drug can't prevent a disease from becoming worse in a person with stable disease. Until recently, it was difficult to identify people at high risk for disease progression. This uncertainty is normally addressed by studying more patients for more time to ensure you have enough of the right patients to show the drug's true benefits if it works.
Polygenic risk scores are helping to reduce these uncertainties. Polygenic means many genes, and a polygenic score calculates risk based on the collective effects of numerous common genetic variants in DNA—up to millions of variants in some cases. This approach has shown that people who appear to be at low to moderate risk, based on standard diagnostic tests, may actually be at serious risk for disease progression.
"A drug's treatment effect should emerge sooner and more clearly in patients at greater risk for poor outcomes," said Lenz. "At Amgen, we're using human genetics and related data to reduce the size and duration of our trials, increase the probability of success and the size of the treatment effect, and get our medicines approved and into the hands of the patients who need them much sooner."
Applying digital innovation to execute faster trials
The director Alfred Hitchcock once said that actually making a movie was boring compared to planning the whole film in his mind. A similar perspective has long held sway with regard to running clinical trials. "Traditionally, the actual execution of clinical trials has not been viewed as an innovation-rich activity," said Lenz. "But that is changing."

Rob Lenz, senior vice president, Global Development
"The speed of a trial largely depends on getting patients enrolled as quickly as possible, which in turn depends on building a network of high-performing study sites," said Taras Carpiac, executive director, Process Improvement. "The traditional way to select study sites has been to send surveys to potential investigators and say, 'Here's the rough design of our study. Would you like to participate? And how many patients do you think you can enroll?' With that approach, we can only take a few factors into account, like how many patients an investigator has enrolled in previous trials."
Predictions based on a handful of factors may not be very accurate, said Julie Argento, director, Global Clinical Program Management. "Across the industry, roughly 15% of sites that sign up for a trial do not enroll a single patient, and that can be higher for some disease indications." The resulting lag slows down the entire trial.
To get better predictions, Amgen is turning to digital innovation and artificial intelligence. "By implementing a machine learning approach, we can now consider hundreds of characteristics when selecting study sites," said Argento. "We can identify key drivers of enrollment, including specific patient populations in different geographies, a site's research capabilities and infrastructure, available staff, experience with genomic screening, and so on. This allows us to differentiate sites that are likely to be strong enrollers as well as sites predicted to be non-enrollers."
Lowering burdens to boost diversity
Richer analytical data can also help to pinpoint aspects of a study's design that might shrink the number of patients who qualify or discourage patients or investigators from participating. "Industry-wide, an alarmingly high percentage of investigators never choose to do a second trial," said Carpiac. "It can be too much of a hassle for a medical practice that isn't built around running trials."
The extra work—recruiting and evaluating patients, in-depth diagnostic tests, collecting, inputting, and cleaning data, etc.—can overwhelm an already busy healthcare practice. These burdens can be eased by simplifying the protocol as much as possible to generate the needed data and by collecting much of the data directly from the electronic medical records of study participants. "The idea is to design the study so that it fits more seamlessly into standard medical practice," said Carpiac.
Patient-centric study designs can also speed up recruitment by making it easier to participate. According to industry metrics, patients enrolled in trials travel more than 50 miles roundtrip on average for each clinic visit. "These are often people whose lifestyle is already severely compromised by disease," Carpiac noted. Reducing the number, duration, or difficulty of site visits can lower barriers that dissuade patients from enrolling in trials or cause them to drop out of a study after it starts.
Toward that end, Amgen is making greater use of decentralized approaches wherever feasible. Some of these solutions began as necessities due to the COVID-19 pandemic, but they are now being embraced as ways to speed up enrollment in trials by making them more patient-friendly, said Carpiac. "Decentralization can mean anything from home healthcare visits and telemedicine to collecting data through wearable devices or shipping clinical supplies directly to patients' homes."
Reducing participation burdens on patients as well as sites can also advance the goal of improving clinical trial diversity. Democratized trials can expand the clinical research landscape beyond large academic health centers to encompass more community-based practices, while ensuring that the participants in a study are more characteristic of the broader patient population.
Historically, Amgen has excelled at delivering first-in-class medicines. Of the 20 innovative therapies in Amgen's product portfolio, 13 have earned a first-in-class designation and three more are based on earlier first-in-class mechanisms developed at Amgen. That track record will be more challenging to maintain going forward.
"The good news for patients is that we're seeing more investment than ever in biotechnology, which is leading to the discovery of more potential new medicines," said Lenz. "But that also means unprecedented levels of competition within the industry, with multiple companies pursuing nearly every drug target that looks promising. Scientific innovation is still essential, but speed is becoming more and more important to the success of any new therapy."
"The mRNA vaccines developed in response to the pandemic has shown us what can be done and what the edge of achievability looks like," said Reese. "That's what we should be striving to achieve every day."