Experience the interactive Multimedia News Release here: https://www.multivu.com/players/English/74140512-amgen-postmenopausal-osteoporosis-study.
"Treatment data show that only one in five women who have experienced an osteoporotic fracture are started on treatment for the disease1, despite the fact that patients who experience an osteoporotic fracture are twice as likely to suffer a future fracture2," said study lead author
FRAME (FRActure study in postmenopausal woMen with ostEoporosis), which enrolled 7,180 women, showed that those randomly assigned to receive a monthly subcutaneous 210 mg dose of romosozumab experienced a statistically significant 73 percent reduction in the relative risk of a new vertebral (spine) fracture through 12 months, the first co-primary endpoint, compared to those receiving placebo (fracture incidence 0.5 percent versus 1.8 percent, respectively [p<0.001]). Of interest, the data showed that by six months, new vertebral fractures occurred in 14 romosozumab and 26 placebo patients, and between six to 12 months, fractures occurred in two additional romosozumab patients versus 33 additional placebo patients.
For those patients who received romosozumab in year one, fracture risk reduction persisted through month 24 after both groups transitioned to denosumab treatment in the second year of the study; there was a statistically significant 75 percent reduction in the risk of vertebral fracture at month 24 (the other co-primary endpoint) in patients who received romosozumab followed by denosumab versus placebo followed by denosumab (fracture incidence 0.6 percent versus 2.5 percent, respectively [p<0.001]). In the second year of the study, new vertebral fractures occurred in five patients who transitioned from romosozumab to denosumab and 25 patients who transitioned from placebo to denosumab.
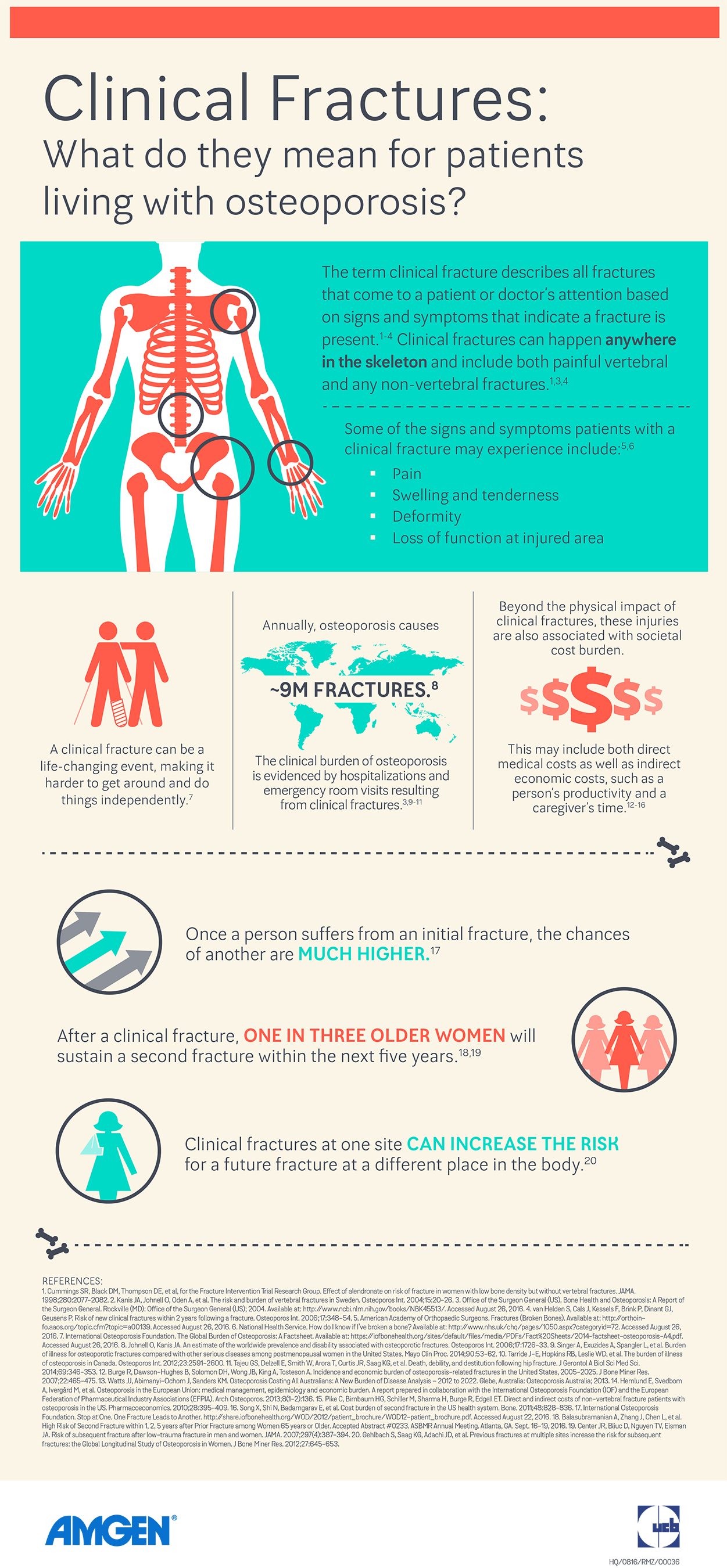
When looking at clinical fractures, which encompass all symptomatic fractures (both non-vertebral and painful vertebral fractures), patients receiving romosozumab experienced a statistically significant 36 percent reduction in the relative risk of a clinical fracture, a secondary endpoint, through 12 months compared to those receiving placebo (fracture incidence 1.6 percent versus 2.5 percent, respectively [p=0.008]). A 33 percent reduction in relative risk of clinical fracture was observed through 24 months after patients transitioned from romosozumab to denosumab compared to patients transitioning from placebo to denosumab (nominal p=0.002, adjusted p=0.096).
Romosozumab resulted in a 25 percent reduction compared to placebo in the relative risk of non-vertebral fractures through month 12, another secondary endpoint, but the reduced risk was not statistically significant (fracture incidence 1.6 percent versus 2.1 percent, respectively, [p=0.096]). For the non-vertebral fracture endpoint, the overall fracture incidence in the study was lower than expected (2.1 percent in the placebo group in year one versus an expected rate of 3.5 percent).
In a sub-study of 126 subjects, romosozumab increased bone mineral density with gains of 9.7 percent and 4.7 percent from baseline by six months at the lumbar spine and total hip, respectively, and gains of 13.3 percent and 6.8 percent at 12 months (all comparisons versus placebo p<0.001). Bone mineral density continued to increase in the romosozumab group after transitioning to denosumab, reaching 17.6 percent and 8.8 percent increases from baseline at the lumbar spine and total hip, respectively, at 24 months (p<0.001 compared to placebo-to-denosumab group for all comparisons).
"We are pleased to see nearly 15 years of sclerostin antibody research reinforced with these Phase 3 data. Romosozumab, with its dual effect as a bone builder and anti-resorptive, has the potential to play a distinct and important role in the treatment of women with postmenopausal osteoporosis at increased risk of fracture," said
"Osteoporotic fractures are common, resulting in far-reaching consequences for individuals and their families, as well as for society as a whole," said Dr.
The percentage of patients with adverse events and serious adverse events in the 12-month double-blind period and 24-month study period were balanced overall between the treatment groups. Injection site reactions, mostly mild in severity, were reported in 5.2 percent of patients in the romosozumab treatment group and 2.9 percent in the placebo group during the 12-month period. There were two positively adjudicated events of osteonecrosis of the jaw in the romosozumab treatment group, one after completing romosozumab dosing and the other after completing romosozumab treatment and receiving the initial dose of denosumab. There was one positively adjudicated event of atypical femoral fracture after three months of romosozumab treatment. Adjudicated serious cardiovascular events and cardiovascular deaths were balanced between treatment groups.
About Romosozumab
Romosozumab is an investigational bone-forming monoclonal agent and is not approved by any regulatory authority for the treatment of osteoporosis. It is designed to work by inhibiting the activity of the protein sclerostin and has a dual effect on bone, both increasing bone formation and decreasing bone breakdown. Romosozumab is being studied for its potential to reduce the risk of fractures in an extensive global Phase 3 program. This program includes two large fracture trials comparing romosozumab to either placebo or active comparator in more than 10,000 postmenopausal women with osteoporosis.
About the FRAME study
FRAME is a multicenter, international, randomized, double-blind, placebo-controlled, parallel-group study in postmenopausal women with osteoporosis, defined as low bone mineral density at the total hip or femoral neck. The study evaluated the effectiveness of romosozumab treatment, compared with placebo, in reducing the risk of new vertebral fractures through 12 months. The study also further evaluated if romosozumab treatment for 12 months followed by denosumab treatment for 12 months, compared with placebo followed by denosumab treatment, was effective in reducing the risk of new vertebral fractures through 24 months. In addition, clinical fracture (a composite endpoint which encompasses all symptomatic fractures, both non-vertebral and painful vertebral fractures) risk reduction, non-vertebral fracture (fractures outside of the spine, excluding sites that are not considered osteoporotic, fractures due to high trauma or pathologic fractures) risk reduction and other endpoints were assessed at 12 and 24 months.
7,180 patients were randomized 1:1 to receive either 210 mg romosozumab subcutaneous (SC) monthly (QM) or placebo SC QM for the 12-month double-blind study period. After the placebo-controlled study period, patients entered the open-label phase where all patients received 60 mg denosumab SC every six months (Q6M) for 12 months, while remaining blinded to initial treatment. An additional 12 month extension period of open-label 60 mg denosumab SC Q6M is currently ongoing.
About Prolia® (denosumab)
Prolia is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy. In postmenopausal women with osteoporosis, Prolia reduces the incidence of vertebral, nonvertebral, and hip fractures.
Prolia is indicated for treatment to increase bone mass in men with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.
Prolia is indicated as a treatment to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer. In these patients Prolia also reduced the incidence of vertebral fractures.
Prolia is indicated as a treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
Important Safety Information (U.S.)
Contraindications
Prolia® is contraindicated in patients with hypocalcemia. Pre‐existing hypocalcemia must be corrected prior to initiating Prolia®. Prolia® is contraindicated in women who are pregnant and may cause fetal harm. Prolia® is contraindicated in patients with a history of systemic hypersensitivity to any component of the product. Reactions have included anaphylaxis, facial swelling and urticaria.
Same Active Ingredient
Prolia® contains the same active ingredient (denosumab) found in XGEVA®. Patients receiving Prolia® should not receive XGEVA®.
Hypersensitivity
Clinically significant hypersensitivity including anaphylaxis has been reported with Prolia®. Symptoms have included hypotension, dyspnea, throat tightness, facial and upper airway edema, pruritus, and urticaria. If an anaphylactic or other clinically significant allergic reaction occurs, initiate appropriate therapy and discontinue further use of Prolia®.
Hypocalcemia
Hypocalcemia may worsen with the use of Prolia®, especially in patients with severe renal impairment. In patients predisposed to hypocalcemia and disturbances of mineral metabolism, clinical monitoring of calcium and mineral levels is highly recommended within 14 days of Prolia® injection. Adequately supplement all patients with calcium and vitamin D.
Osteonecrosis of the Jaw (ONJ)
ONJ, which can occur spontaneously, is generally associated with tooth extraction and/or local infection with delayed healing, and has been reported in patients receiving Prolia®. An oral exam should be performed by the prescriber prior to initiation of Prolia®. A dental examination with appropriate preventive dentistry is recommended prior to treatment in patients with risk factors for ONJ such as invasive dental procedures, diagnosis of cancer, concomitant therapies (e.g., chemotherapy, corticosteroids, angiogenesis inhibitors), poor oral hygiene, and co‐morbid disorders. Good oral hygiene practices should be maintained during treatment with Prolia®. The risk of ONJ may increase with duration of exposure to Prolia®.
For patients requiring invasive dental procedures, clinical judgment should guide the management plan of each patient. Patients who are suspected of having or who develop ONJ should receive care by a dentist or an oral surgeon. Extensive dental surgery to treat ONJ may exacerbate the condition. Discontinuation of Prolia® should be considered based on individual benefit‐risk assessment.
Atypical Femoral Fractures
Atypical low‐energy, or low trauma fractures of the shaft have been reported in patients receiving Prolia®. Causality has not been established as these fractures also occur in osteoporotic patients who have not been treated with anti‐resorptive agents.
During Prolia® treatment, patients should be advised to report new or unusual thigh, hip, or groin pain. Any patient who presents with thigh or groin pain should be evaluated to rule out an incomplete femur fracture. Interruption of Prolia® therapy should be considered, pending a risk/benefit assessment, on an individual basis.
Serious Infections
In a clinical trial (N=7,808) in women with postmenopausal osteoporosis, serious infections leading to hospitalization were reported more frequently in the Prolia® group than in the placebo group. Serious skin infections, as well as infections of the abdomen, urinary tract and ear were more frequent in patients treated with Prolia®.
Endocarditis was also reported more frequently in Prolia®‐treated patients. The incidence of opportunistic infections and the overall incidence of infections were similar between the treatment groups. Advise patients to seek prompt medical attention if they develop signs or symptoms of severe infection, including cellulitis.
Patients on concomitant immunosuppressant agents or with impaired immune systems may be at increased risk for serious infections. In patients who develop serious infections while on Prolia®, prescribers should assess the need for continued Prolia® therapy.
Dermatologic Adverse Reactions
In the same clinical trial in women with postmenopausal osteoporosis, epidermal and dermal adverse events such as dermatitis, eczema and rashes occurred at a significantly higher rate with Prolia® compared to placebo. Most of these events were not specific to the injection site. Consider discontinuing Prolia® if severe symptoms develop.
Musculoskeletal Pain
Severe and occasionally incapacitating bone, joint, and/or muscle pain has been reported in patients taking Prolia®. Consider discontinuing use if severe symptoms develop.
Suppression of Bone Turnover
In clinical trials in women with postmenopausal osteoporosis, Prolia® resulted in significant suppression of bone remodeling as evidenced by markers of bone turnover and bone histomorphometry. The significance of these findings and the effect of long‐term treatment are unknown. Monitor patients for these consequences, including ONJ, atypical fractures, and delayed fracture healing.
Adverse Reactions
The most common adverse reactions (>5% and more common than placebo) in women with postmenopausal osteoporosis are back pain, pain in extremity, musculoskeletal pain, hypercholesterolemia, and cystitis. The most common adverse reactions (> 5% and more common than placebo) in men with osteoporosis are back pain, arthralgia, and nasopharyngitis. Pancreatitis has been reported with Prolia®.
In women with postmenopausal osteoporosis, the overall incidence of new malignancies was 4.3% in the placebo group and 4.8% in the Prolia® group. In men with osteoporosis, new malignancies were reported in no patients in the placebo group and 4 (3.3%) patients in the Prolia® group. A causal relationship to drug exposure has not been established.
The most common (per patient incidence ≥ 10%) adverse reactions reported with Prolia® in patients with bone loss receiving ADT for prostate cancer or adjuvant AI therapy for breast cancer are arthralgia and back pain. Pain in extremity and musculoskeletal pain have also been reported in clinical trials. Additionally, in Prolia®‐treated men with nonmetastatic prostate cancer receiving ADT, a greater incidence of cataracts was observed.
Denosumab is a human monoclonal antibody. As with all therapeutic proteins, there is potential for immunogenicity.
Prolia® Postmarketing Active Safety Surveillance Program
The surveillance program is available to collect information from prescribers on specific adverse events. Please see www.proliasafety.com or call 1‐800‐772‐6436 for more information.
For more information, please see the Prolia Important Safety Information, Prescribing Information, and Medication Guide.
About the
Since 2004,
About
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world's leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
About UCB
UCB,
Forward-Looking Statements –
This news release contains forward-looking statements that are based on the current expectations and beliefs of
No forward-looking statement can be guaranteed and actual results may differ materially from those
The scientific information discussed in this news release relating to new indications for
Forward-Looking Statements - UCB
This press release contains forward-looking statements based on current plans, estimates and beliefs of management. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial information, expected legal, political, regulatory or clinical results and other such estimates and results. By their nature, such forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and assumptions which could cause actual results to differ materially from those that may be implied by such forward-looking statements contained in this press release. Important factors that could result in such differences include: changes in general economic, business and competitive conditions, the inability to obtain necessary regulatory approvals or to obtain them on acceptable terms, costs associated with research and development, changes in the prospects for products in the pipeline or under development by UCB, effects of future judicial decisions or governmental investigations, product liability claims, challenges to patent protection for products or product candidates, changes in laws or regulations, exchange rate fluctuations, changes or uncertainties in tax laws or the administration of such laws and hiring and retention of its employees. UCB is providing this information as of the date of this press release and expressly disclaims any duty to update any information contained in this press release, either to confirm the actual results or to report a change in its expectations.
There is no guarantee that new product candidates in the pipeline will progress to product approval or that new indications for existing products will be developed and approved. Products or potential products which are the subject of partnerships, joint ventures or licensing collaborations may be subject to differences between the partners. Also, UCB or others could discover safety, side effects or manufacturing problems with its products after they are marketed.
Moreover, sales may be impacted by international and domestic trends toward managed care and health care cost containment and the reimbursement policies imposed by third-party payers as well as legislation affecting biopharmaceutical pricing and reimbursement.
CONTACT:
CONTACT: UCB,
T +32.2.559.9178, france.nivelle@ucb.com
T+32.2.559.92.64, Laurent.schots@ucb.com
T +32.2.559.94.14, antje.witte@ucb.com
Isabelle Ghellynck, Investor Relations, UCB
T+32.2.559.9588, isabelle.ghellynck@ucb.com
1. Wilk A et al. Post-fracture pharmacotherapy for women with osteoporotic fracture: analysis of a managed care population in the
2.


To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/results-from-phase-3-frame-study-of-romosozumab-showed-significant-reductions-in-both-new-vertebral-and-clinical-fractures-in-postmenopausal-women-with-osteoporosis-300329792.html
SOURCE